
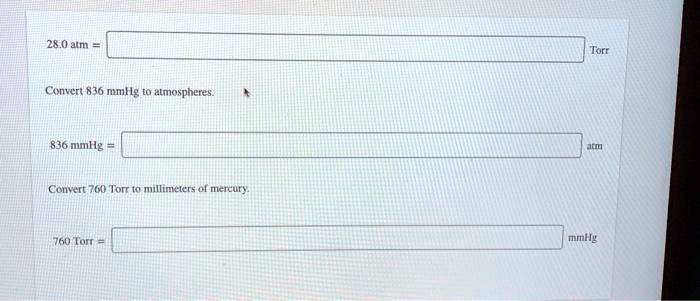
old women is normally distributed with a mean of 120 mmHg (millimeters of Mercury) and a standard deviation of 14 mmHg. Use the empirical rule to solve the problem. If a sample of Helium gas is collected over water, what is the pressure of the dry gas alone if the total pressure is 760.0 mmHg and the partial pressure of water is 20.0 mmHg? What is the molecular weight of a gas if a 15.0g sample has a pressure of 836 mm Hg at 25.0 degrees C in a 2.00 L flask? a)167 b)1.35 c)176 d)11.1 e)none of the above Please explain. If the balloons original volume was 1.05 liters, what will the new volume be at a higher altitude, where the pressure is only 625 mmHg? Assume the temperature stays the According to Chebyshev's Theorem, at least what percentage of the islanders have bloodĪ balloon is filled with hydrogen at a temperature of 20 C and a pressure of 755 mmHg.

Suppose that the blood pressure of the human inhabitants of a certain Pacific island is distributed with mean, \mu = 108 mmHg and standard deviation, \sigma = 6 mmHg. Maths *have answer, want method* Urgent!.If 12.20 g of potassium chloride are dissolved in 200.0 g of methanol, what is the vapor pressure of the solution? A.123.9 mmHg B.3.256 mmHg C.104.3 mmHg D142.7 mmHg The vapor pressure of pure methanol at 25 degrees Celsius is 127.2 mm Hg. Ag2CO3(aq) + 2HCl(aq) ¡÷ 2AgCl(s) + CO2(g) + H2O(l) The carbon dioxide generated is collected in a 19 mL vial and exerts a pressure of A 500 mL saturated silver carbonate solution at 5¢XC is treated with hydrochloric acid to decompose the compound. If the vapour pressure of water at 97.5˚C is 690 mmHg, what percent (by mass) of theĢ. The boiling point of limonene is 175 ˚C, but it co-distills with water at 97.5 ˚C. Limone (MW 136g/mol) is a pleasant smelling liquid found in lemon and orange peels. Using the empirical rule, what percentage of adult males have diastolic blood pressure readings Suppose that diastolic blood pressure readings of adult males have a bell-shaped distribution with a mean of 84 84 mmHg and a standard deviation of 9 9 mmHg. Solve for the elevation at which the pressure is 380 mmHg. The earth temperature at ground level is 11.33℃ and the pressure is 760 mmHg. The temperature of the earth’s atmosphere drops about 5℃ for every 1000m of elevation above earth's surface. Calculate the partial pressure of isopropyl alcohol above a solution in which the mole fraction of propyl alchol is 0.250. The Vapor pressures of pure propyl alcohol and isopropyl alcohol are 21.0 mmHg and 45.2 mmHg, respectively, at 25 (degrees)C. Thank you so much!!!! A student stands on a bathroom scale in an elevator at rest on the 64th floor of a building. I've been staring at it for about an hour now and I have no clue where to even start. I'm sorry this is kind of a long question. What was the temperature of the propane in the flask. After the sample was collected, the gas pressure was found to be 741 mmHg. What is the minimum volume of water required to isolate 10 g of aniline in a steam distillation at 760 mmHg?Ī 2.50 L flask was used to collect a 5.65 g sample of propane gas, C3H8. The vapor pressure of water at 98.2 C is 717 mmHg. The balloon ascends to an altitude of 20 km, where the pressure is 76.0 mmHg andĪniline (MW93 g/mol) co-distills with water 98.2 C. The initial volume of the balloon is 5.00 L, and the pressure is 760. What is the final pressure in the flask? 2CO2 (g) + O2 (g) -> 2CO2 (g) I thought it was just a simple addition, but it wasĪ very flexible helium-filled balloon is released from the ground into the air at 20. if the pressure of co2 is 357 mmhg measured at the same temperature and volume as the original mixture, calculate the mole fraction ofĬarbon monoxide at a pressure of 1490 mmHg reacts completely with O2 at a pressure of 745 mmHg in a sealed vessel to produce CO2. The gases are burned in air to form co2 and h2o. Not sure of formula for thisĪ mixture of methane and ethane is stored in a container at 294 mmHg. What is the pressure of the nitrogen gas if the atmospheric pressure is 744 mmHg? Answer in units of mmHg. Assume the air is an ideal gas withĪ sample of nitrogen gas is collected overwater at a temperature of 23◦C. If the air temperature at ground level is 15☌ and 760 mmHg, determine the elevation in m when the pressure is 380 mmHg. The temperature of earth atmosphere drops 5☌ for every 1km elevation above the earth surface. Assume ideal behavior and calculate the partial pressures of ethanol and 1-propanol at 35 degrees Celsius over a solution of ethanol in 1-propanol, in The vapor pressures of ethanol (C2H5OH) and 1-propanol (C3H7OH) are 100 mmHg and 37.6 mmHg, respectively.


 0 kommentar(er)
0 kommentar(er)
